I describe the three steps of my alternative model to the Plan-Do-Study-Act (PDSA) model for quality assurance/quality improvement (QA/QI) in healthcare.
Category Archives: Quality Assurance/Quality Improvement (QA/QI)
Read my last post in a series on data-related misconduct at startup Theranos outlined in the book, “Bad Blood”, where I discuss their lack of administrative barrier between research and clinical data.
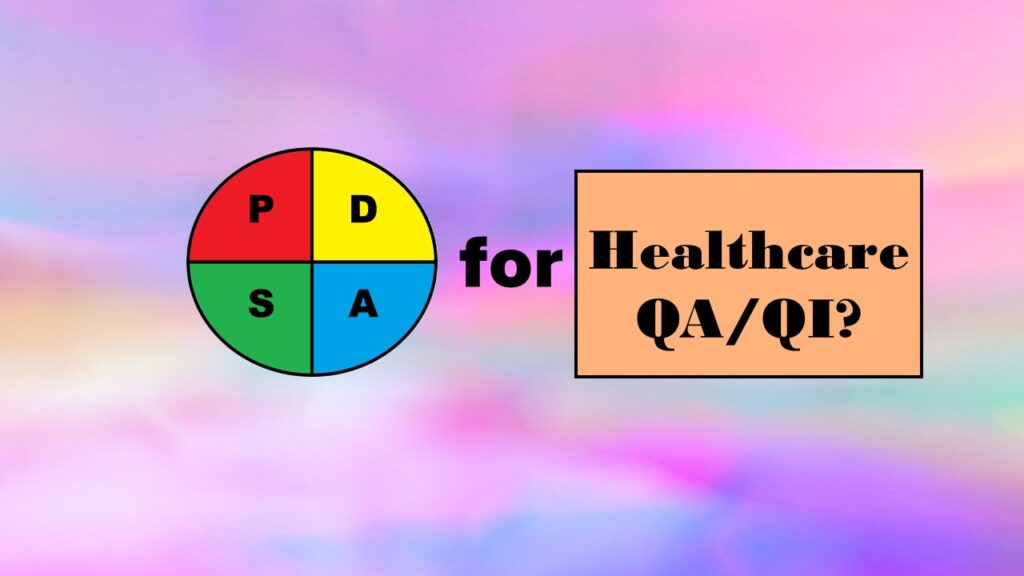
Want an alternative to the Plan-Do-Study-Act (PDSA) model for quality assurance/quality improvement (QA/QI) in healthcare? I recommend approaching QA/QI a different way, by thinking about the various functions of the QA/QI department.
As a data science leader, what should you put in place so your organization doesn’t end up a data mess like startup Theranos? This blog posts provides guidance.
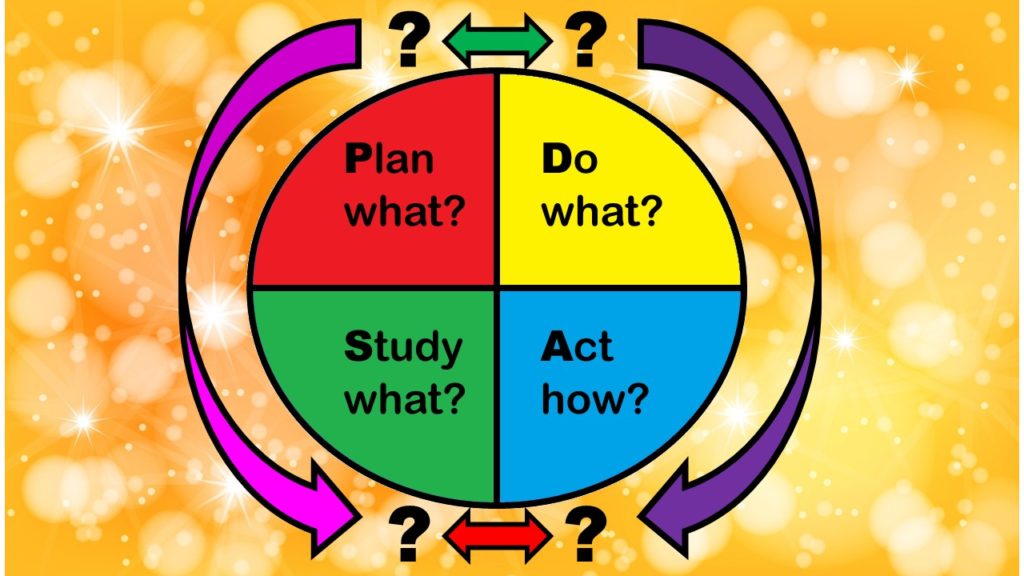
The Plan-Do-Study-Act model is promoted for quality assurance/quality improvement in healthcare. But does it have any peer-reviewed evidence base behind it? I examine that in this blog post.
The book “Bad Blood” describes the fall of startup unicorn Theranos, but also provides insight into the company’s abject failure at data stewardship, which I talk about in this blog post.
This blog post talks about how lack of product description led to data-related misconduct at Theranos, because they could never nail down exactly what they were trying to do.
What are the stages of the PDSA model, and how do they relate to the functions of a QA/QI department in healthcare? The answers are not straightforward. I examine these issues in this blog post.
Wondering what the Plan-Do-Study-Act (PDSA) Model is, and if you should adopt it for quality improvement in healthcare? Read my series of blog posts on the subject for my personal experience and recommendations
This is my first blog post in a series of five where I talk about data-related misconduct outlined in the book “Bad Blood”, and provide guidance on how to prevent it.